Becoming a Force in Digital Health.
RF Health Press/News

The MAC System Earns Winner Title in Renowned 2024 Edison Awards
The Edison Awards™, named after the American inventor Thomas Alva Edison, recognizes some of the most innovative products and business leaders in the world including Steve Jobs, Gwynne Shotwell, Ginni Rometty and Lanzatech, Apple, and Lockheed Martin. The prestigious accolade honors excellence in new product and service development, marketing, human-centered design, and innovation.
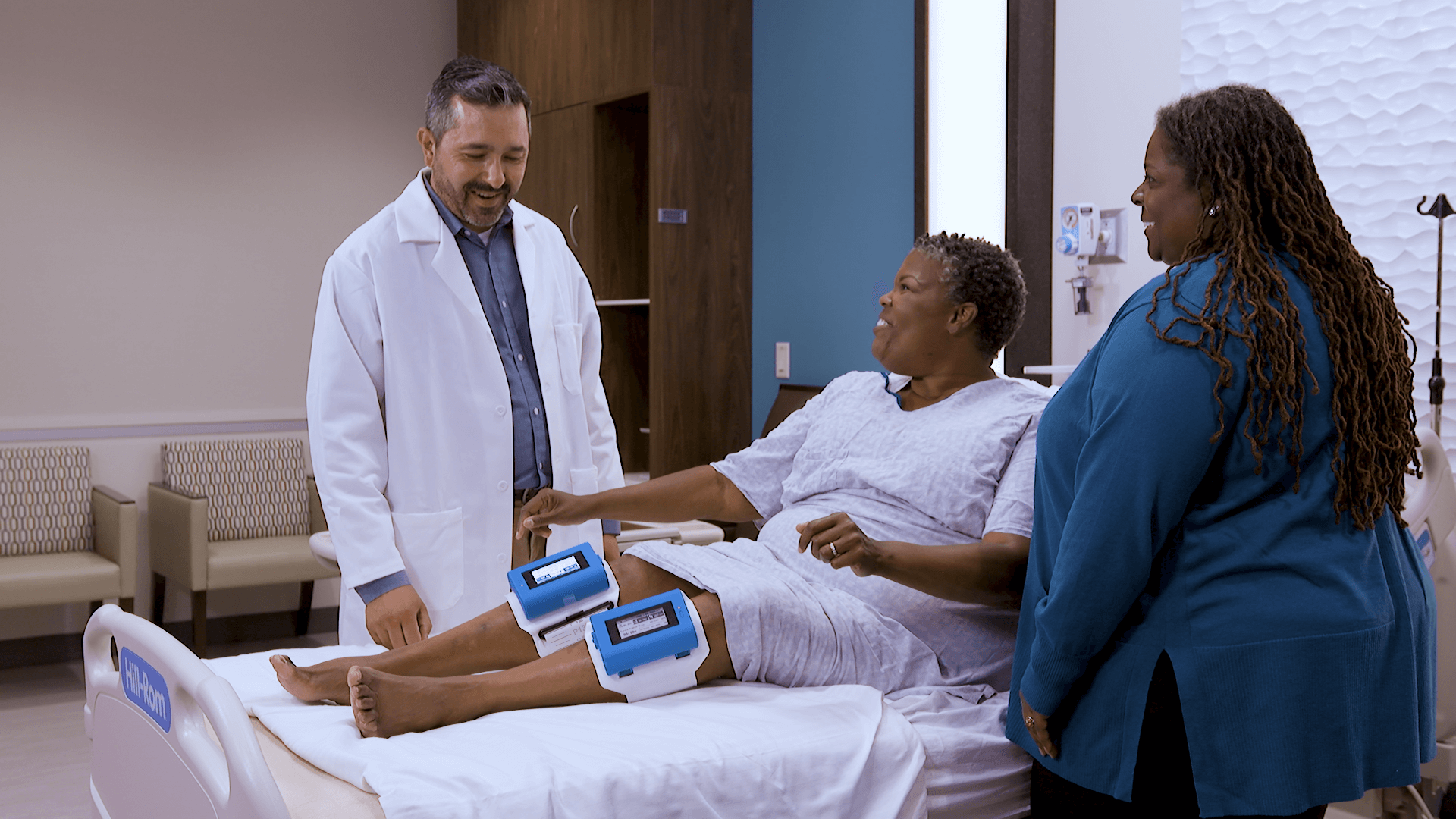
Recovery Force Health awarded Vascular Compression Therapy agreement with Premier, Inc.
Premier is a leading healthcare improvement company, uniting an alliance of approximately 4,350 U.S. hospitals and 300,000 other providers to transform healthcare. With integrated data and analytics, collaboratives, supply chain solutions, consulting and other services, Premier enables better care and outcomes at a lower cost.

Recovery Force Health Selected as a Nominee for Two ‘Best of Tech’ TechPoint Mira Awards
TechPoint, the industry-led growth initiative for Indiana’s digital innovation economy and overall tech ecosystem, announced the nominees for its 25th annual Mira Awards program on February 20th. The Mira Awards are the state’s largest, best known and most prestigious technology awards, and the annual gala, taking place in April, celebrates the “Best in Tech” in Indiana.

Recovery Force Health’s Movement And Compressions (MAC) System Named as a Finalist in the World-Renowned 2024 Edison Awards™
The Edison Awards™, named after the American inventor Thomas Alva Edison, recognizes some of the most innovative products and business leaders in the world including Steve Jobs, Gwynne Shotwell, Ginni Rometty and Lanzatech, Apple, and Lockheed Martin. The prestigious accolade honors excellence in new product and service development, marketing, human-centered design, and innovation.

Recovery Force Health Announces Government Channel Partner Agreement with Marathon Medical
Fishers, Indiana, September 15, 2023, Recovery Force Health is an all-encompassing digital health company focused on the development of data-driven solutions through wearable medical technology.has been announced as a winning company in The 2023 Powderkeg Top 100 Unvalley Awards. This recognizes Recovery Force Health as one of the best tech companies to work for in emerging tech communities beyond Silicon Valley.

Recovery Force Health Named a 2023 Powderkeg Top 100 Award Winner
Fishers, Indiana, September 15, 2023, Recovery Force Health is an all-encompassing digital health company focused on the development of data-driven solutions through wearable medical technology.has been announced as a winning company in The 2023 Powderkeg Top 100 Unvalley Awards. This recognizes Recovery Force Health as one of the best tech companies to work for in emerging tech communities beyond Silicon Valley.

Recovery Force Health Selected to Exhibit the Movement And Compressions (MAC) System at the 2023 Vizient Innovative Technology Exchange
Fishers, Indiana, June 7, 2023— Recovery Force Health has been selected to exhibit the Movement and Compressions (MAC) System which is a cordless, tubeless, lithium-ion powered device that measures real-time patient mobility data while simultaneously delivering therapeutic compressions to prevent blood clots at the Vizient Innovative Technology Exchange

Medtech Company Recovery Force Health Secures $1.25M in Funding
Indianapolis, Ind. (March 9, 2023) – Recovery Force Health, a medical technology company focused on data-driven, wearable devices, is announcing today the close of a $1.25 million investment round with Elevate Ventures.

Recovery Force Health Presents At The 2023 State of Medical Device True Quality Summit Series
Greenlight Guru's annual 2023 State of Medical Device Industry Report is coming to help you understand the strategies, tactics, and technologies used today to accelerate product development, ensure compliance, and promote quality.
Get a first look into trends, outlooks, statistics, and opportunities in the medical device industry from data gathered from 600+ medical device professionals at this upcoming True Quality Summit Series.

Indiana-based Recovery Force Health Launches Innovative Medical Device
Recovery Force Health has developed a therapeutic, cordless VTE compression device that also tracks patient positioning and mobility. This device is a gamechanger for bedside caregivers in hospitals to collaborate and adhere to mobility protocols. Segment produced by Inside Indiana Business.

Recovery Force Health to Commercially Launch Movement And Compressions at NTI 2022
Recovery Force Health’s Mobility Measurement device provides therapeutic compressions to prevent DVT. Meet the MAC System.

Recovery Force Presents At The 2021 American Heart Association’s Scientific Sessions
Recovery Force, LLC has been selected for a showcase presentation during the 2021 American Heart Association Annual (Virtual) Scientific Sessions, the world-leading annual event for researchers, medical and healthcare professionals in science, prevention, and treatment of cardiovascular pathologies.

Movement And Compressions (MAC) featured in the 2021 Journal of Vascular Surgery
The following open access version of a recently published article Comparison of a Non-Pneumatic Device to Four Currently Available Intermittent Pneumatic Compression Devices on Common Femoral Blood Flow Dynamics is now available online.

Mobility measurement and therapeutic device receives landmark FDA Clearance - introducing the MAC System
FISHERS, Ind., March 22, 2021 /PRNewswire/ -- Recovery Force, LLC is pleased to announce the company has received U.S. Food and Drug Administration (FDA) 510(k) clearance for the use of the Movement and Compressions (MAC) System, a novel, data-driven, mobility measurement device designed for hospital and home use.

Recovery Force Health At Home
Second Round of Manufacturing Readiness Grants Awarded to 31 Indiana Businesses
RF Health: The Indiana Economic Development Corporation (IEDC), in partnership with Conexus Indiana, announced the second round of awards totaling approximately $3 million to 31 Indiana businesses in Manufacturing Readiness Grants, which Governor Eric J. Holcomb first announced in May. The grants are part of the Economic Activity Stabilization and Enhancement (EASE) program designed to stimulate manufacturing investments that will position Hoosier operations, and the sector overall, for future growth and prosperity.
Recovery Force ISO 13485 Certification
ISO 13485, Medical devices – Quality Management Systems – requirements for regulatory purposes, is an internationally recognized standard for organizations involved in the medical device industry. Companies are using compliance to this standard to obtain the certification of their Quality Management System. The primary purpose of the ISO 13485 standard is the Harmonization of the Medical Device regulatory requirements for Quality Management Systems.
$1.8 million NIH grant funds IU researcher’s work on mobile compression device
Recovery Force’s Mobile Active Compressions (MAC) calf device has been in development since the company was founded five years ago. Now, a $1.8 million grant from the National Institutes of Health will cover the cost of testing the device on some 300 patients in Indianapolis and Boston over the next two years.
Recovery Force Receives Prestigious $1.8M Grant from National Institutes of Health
Funds will aid development and clinical validation of company’s flagship product
Mayo Clinic Ventures Partners with Wearable Medtech, Recovery Force
Indiana-based Recovery Force makes microprocessor-controlled compression wraps.
Company Awards / Achievements
-

National Institutes of Health - Phase I Award
Small Business Innovative Research (SBIR) $300k Innovation Grant
Initial funding supports the development of wearable technology to reduce the risk of DVT and increase patient compliance.
-

National Institutes of Health - Phase II Award
Small Business Innovative Research (SBIR) $1.8M Innovation Grant
Validation of wearable technology to reduce the risk of DVT and optimize mobility measurement.
-

Top Cardiac and Vascular Disorder Startup 2021
The Cardiac and Vascular Disorders has over 2.2K+ startups that comprise of companies that engaged in R&D for developing medical devices, diagnostics tests, and pharmaceuticals for the diagnosis and treatment of cardiac and vascular disorders.
Recovery Force made a curated list of the most promising startups leading the Cardiac and Vascular Disorders industry, from across the globe.
+ Disclaimer/Acknowledgement
Research reported in this presentation was supported by the National Heart, Lung, And Blood Institute of the National Institutes of Health under Award Numbers R43HL132624 & R44HL132624. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Grant number R43HL132624 financed jointly with 83 percent government ($244,167) and non-governement ($50,000) funding. Grant number R44HL132624 financed with 100 percent government ($1,763,777) funding.
